Under Graduate Botany (Major ) , 1st Semester. Microbiology#Phycology By Prasenjit Sinha
Botany Major ,NEP 2020,Tripura University.
Unit II, Microbiology II,
1.Significance of Indian Plant Bacteriologist P.Gunasekaran.
Photograph Courtesy: The Biotech Research Society India( BRSI).
Prof.Dr.P.Gunasekaran ,VC,VIT University Bhopal has 32 years of teaching and research experience in Microbiology, Biotechnology and genomics . Metagenomics and Bioprospecting , ko Molecular Biology and Genomics are his research area. His contributions are as follows:
(1) Research Projects: 32
(2) Grants received: ₹5510 lakhs
(3) Workshops & Conferences Organised: 20
(4) Students awarded: Ph.D. 44 ; M.Phil 25
(5) MSc students project: 87
(6) Publications in Journals: 175
(7) Book Chapters written: 30
(8) Papers presented in Conferences : 192
(9) Books written: 20
Dr.PGS has begged several National and International Awards and Fellowships and held many more Positions from Lecturer (1980) - Senior Professor and Head (2007) and VC of VIT, Bhopal,India.
2.Bacteria
A bacterium is a prokaryotic single celled organism and considered as simplest of all organisms as it lacks membrane bound cell organelles like nucleus,mitochondria, etc. Anton von Leeuwenhoek first observed them in 1672, but described as " bacteria" in 1683.
2.1. Archaebacteria
Archaebacteria are the ancient bacteria . They lack murein in their cell wall .These bacteria are divided into five groups :
Part - I - Methanogens: These are the strict anaerobic and they reduce carbon dioxide to methane. e.g. Methanobacterium, Methanococcus, Methanomoctobium, etc.
Part -II- Archae-Sulphate Reduces: Archaeoglobus belongs to this group which grows at 65°C to 90°C and reduces thiosulphate , sulphate, sulphite and sulphide to hydrogen sulphide.
Part-III- Extreme Halophilloc- Aerobic : These are obligate halophiles(15% NaCl) . e.g. Halococcus , Halobacterium , etc.
Part-IV : Cell Wall-less Archae: Thermoplasma is acidophilic (pH -2) , thermophilic (59°C) and grow in both aerobic and anaerobic conditions.
Part - V: Extreme Thermophilic Archae: Members metabolize sulphur, grow at very high temperatures.
e.g. Thermoproteus grow at 85°C - 105°C , Acidianus at extreme pH 2-3.
Pyrodictium is the most Thermophilic which grow at temperature upto 110°C and exhibits it's optimum growth at 105° C [21] .
2.1.1.Characteristics of Archaebacteria
(1) The members of Archaebacteria can live in different habitats, (i) in rumens and termites as anaerobic methanogens, (ii) in extreme saline condition as halophiles which require NaCl , (iII) in thermal vents and hot spring( above 100°C) as thermophiles ,and (iv) as thermoacidophiles in both hot and acidic environment. Archaebacteria are unable to grow at temperature below 60°C.
(2) Cell structure is prokaryotic type.
(3) Size: 0.1 micrometre to 1.5 micrometre in dia., if filamentous 200 micrometre in length.
(4) They may be gm + or gm - .
(5) Some member's cell wall is made up of pseudomurein/ peptidoglycan only. Cell wall lack peptidoglycan, hence, naturally resistant to enzyme lysozyme and beta-lactum antibiotics.Archaeal wall does not have muramic acid and D- amino acids.
(6) Cell membrane lipid of Archaea have ether linked chains of 20 carbons or 40 carbons which enable them to tolerate acidic pH and heat.
(7) Archaeal genome(DNA) is circular and linked covalently.
(8) In Thermoplasma acidophilus genome size is 0.8 x 10⁹ Daltons and 1.1 x 10⁹ in Methanobacterium thermoautotrophicum.
(9) Ribosomes 70 S type.
(10) Number of RNA polymerase ,with 8-12 sub- units , in Archae are many .
(11) Chemolithotrophic.
(12) Promoter have TATA box.
(13) Plasmids present.
2.2. Characteristics of Eubacteria
(1) Prokaryotic,unicellular and size ranges from 0.5 micrometre to 2.0 micrometre.
(2) May be gm+ or gm-.
(3) Muramic acid is present in the cell wall.
(4) Lipids in cell membrane are ester linked.
(5) Number of RNA polymerase is one ; it is made has 4 sub units only.
(6) Bacteria are sensetive to antibiotics.
(7) Promoter have Pribnow box.
(8) Ribosome 70 S type.
(9) They can not grow at or above 80°C.
(10) Plasmids present in them.
Question Bank
1. Define/what are bacteria.
2. What are Archaebacteria?
3.What kind of ribosomes are found in Archaebacteria?
4. Name one ancient Bacteria.
5.What are Methanogens? Give one example.
6. What are Halophiles? Give one example along with the habitat.
7. A member of Archaebacteria can tolarete acidic pH and heat.How?
8. State the characteristics of Archaebacteria.
9. State the features of Eubacteria.
10. Differentiate between Archaebacteria and Eubacteria
11. Name the most Thermophilic Archaebacteria.
12. A bacterium is prokaryotic. Justify.
13. Note on Prokaryotic features of Archaebacteria.
14. Note on Eukaryotic features of Archaebacteria.
2.3. Morphological forms of Bacteria:
Bacterial shape is primarily dictated by the only solid element peptidoglycan sacculus(Salton and Home,1951; Widely et al.,1960) present in the bacterial envelope. Bacillus, Coccus and Spiral are the major forms, in addition more exotic shapes like stars, mustaches,serpentine and branches are the other forms of bacteria ( Young,2006; Kysela et al.,2016) [1] .
[I] Bacillus or rod-shaped bacteria
Greek "Bacillus means stick".Rod-shaped Bacillus is the most common form of bacteria. Their ends may be round or blunt and are motile or non-motile. Average size is 1.5 micro mt in length and 0.5 micro mt in diameter e.g., Bacillus anthracis, Lactobacillus ,etc..
(i) If they occur in pair ,then diplobacillus , e.g., Corynebacterium diphtheriae and
(ii) streptobacillus occur in group in long chains, e.g., Bacillus cereus , Bacillus tuberculosis.
(iii) If the length and width are equal,then such bacilli is known as " coccobacilli" ,e.g., Bracella [2] .
These cells appear hyphens(-) under microscope.
[II] Coccus or spherical Bacteria
Greek" Kokkos "means berry. Spherical Bacteria are termed as Cocci (sing, Coccus) and measure 0.5-2.5 micrometre in diameter.Cocci are non-motile, atrichous and of six types on the basis of number of cells arrange.
(i) Micrococci: When a Coccus appears singly ; e.g., Micrococcus cerolyticus ,M.cryophilus,etc.
(ii) Diplococci: When occur in pairs, e.g., Diplococcus pneumoniae.
(iii) Streptococci : When occur in a chain e.g., Streptococcus lactos, S. pyogenes.
(iv) Tetracocci: When appear as tetrads ( group of four cells) , e.g., Pedicoccus cerevisiae.
(v) Staphylococci: When appear in irregular clusters, e.g., Staphylococcus aureus,S.albus.
(vi) Sarcinae: When appear as a cube due to division of regular three planes, e.g.Sarcinae lutea,S. verticuli.
[III] Spiral or Helical bacteria
This form is least common and the cells of them are spirally curved, hence, called helicoid or curved. These bacteria have one or more flagella at each pole .e.g. Spirilum undulum, S. volutans ,S. minus.
[IV] Vibrios
These bacteria have a slight curve and a comma (,) like appearance having a single flagella at the tip .e.g. Vibrio coli ,V. cholerae.
[V] Filamentous
Some bacteria such as Beggiatoa alba and Thiothrix are filamentous.
[VI] Pleomorphic
Some bacteria are able to change their size and shape in the basis of surroundings ,e.g. Acetobacter may occur as a single rod (bacilus) or chain of some rods ( streptobacillus).
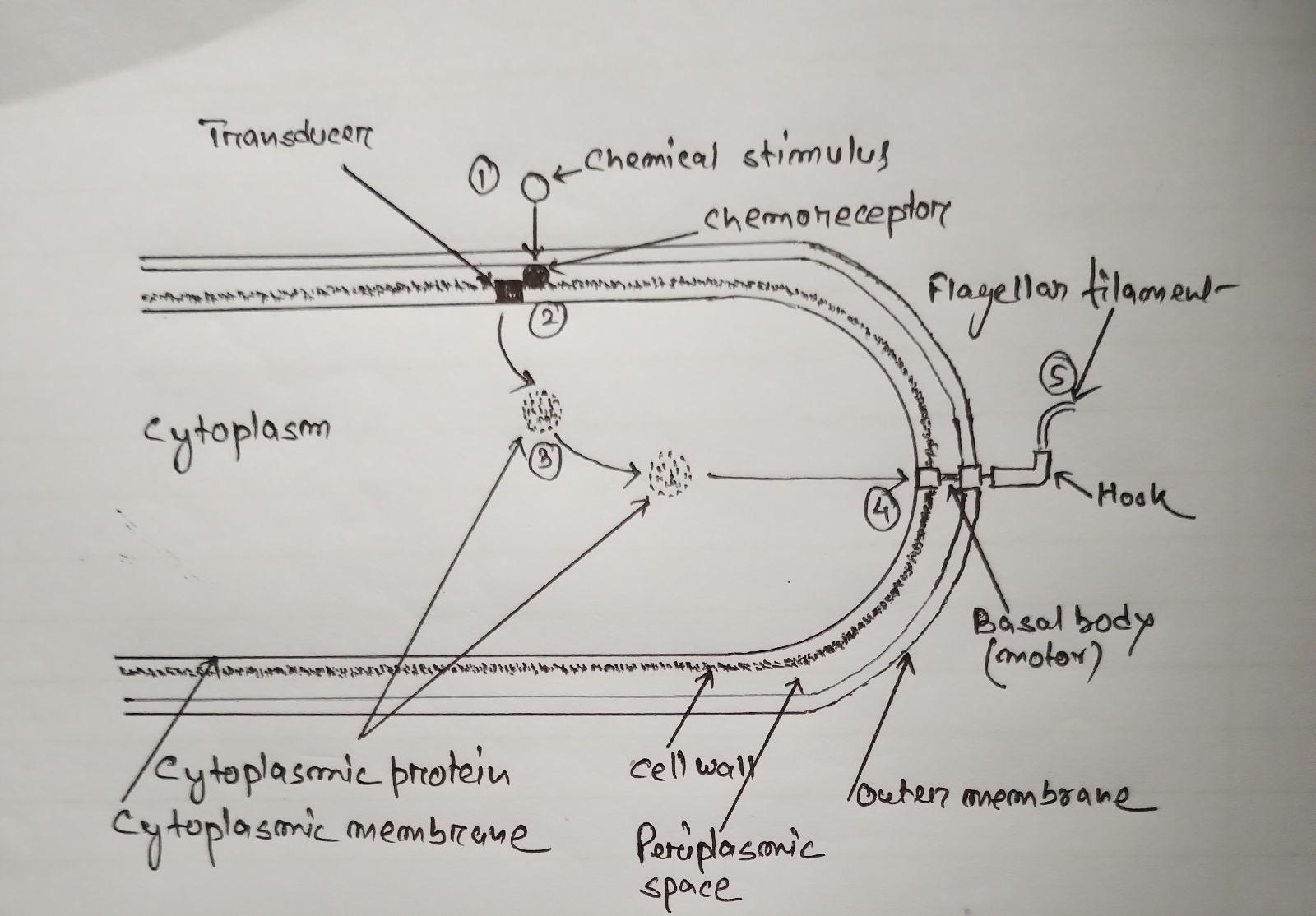
3.Bacterial chemotaxis:
3.1.1. Definition : According to the Biology Online Dictionary, " Chemotaxis is the directional movement of an organism or a living motile cell in response tocertain diffusible chemicals in the environment .It is of two types: (1) Positive Chemotaxis: when the movement is towards a higher concentration of the diffusible substance,and (2) Negative Chemotaxis: i.e., the movement towards the lower concentration of diffusible substance [20] .
3.1.2. Demonstration:
The phenomenon of Chemotaxis can be explained in a simple manner by the experiment performed by Beijerinck . The experiment is as follows:
(2) Allow the bacteria to move to their optimal oxygen concentration (here, the stimulus).
Observation:
(1) Strongly aerobic bacteria collected in large numbers at the margin of coverslip.
(2) Anaerobic bacteria moved to the centre , and
(3) Microaerophilic bacteria collected at some distance from the margin of the coverslip.
Inference:
(1) Bacteria can regulate their flagellar movement in response to the stimulus (in this case,oxygen) either positively or negatively.
(2) Bacteria exhibit a directional movement towards a concentration gradient of the chemical stimulus.
(3) If the medium is homogeneous i.e. without any concentration gradient, then bacteria show non-directional movement.
3.1.3. Mechanism of Chemotaxis in bacteria:
To perform Chemotaxis, bacteria utilise a sensory system which consists of a sensory system having chemoreceptors and transducers.
(1) At first, the chemoreceptor proteins located at the periplasm of gm- bacteria) receive the chemical stimulus on the cell surface.
(2) Next, chemoreceptors transmit a signal to the transducer proteins , located in the cytoplasmic membrane.
(3) Then, the transducers transmit that signal to the flagellar motor through small cytoplasmic proteins. At least two cytoplasmic proteins appear to be involved.
(4) The direction of rotation of the flagellar motor is controlled by methylation and demethylation of the transducer proteins.
(5) Methylation of transducer proteins activate the cytoplasmic protein by phosphorylation.
4. Bacterial Reproduction:
Majority of bacteria reproduce asexually by means of Binary fission, Conidia, Cyst, Budding, and Endospore formation.
4.1: Binary fission:
An unicellular bacterium divides to produce two identical cells.These two offsprings again perform binary fission after attaining maturity.Thus , if the conditions are favourable,then the cell number and mass of bacteria turn twice/double following the geometric progression . The time gap between two successive fission is termed as the generation time / doubling time.
The geometric progression can be represented as 2°-->2¹-->2²-->2³-->2⁴--2⁵........ Bacteria can carry out binary fission process once every 20 minutes when nutrients are enough and temperature is favorable.
e.g. Under suitable conditions, a type of bacterium divides every 20 minutes.Calculate the number of bacteria present after 4 hours .
4 hrs = 240 minutes
The round of bacterial division= 240÷20= 12
So, number of bacteria after 4 hrs= 2¹² = 4096 .
Step I : DNA Duplication: At first two strands of DNA replicate, then divides into two equal halves.
Step II : DNA Partitioning : According to Kleppe et al. 1979, DNA is attached to some point to the plasma membrane, and after DNA Duplication each one of the double strands swings towards one of the cell halves, keeping itself in the same attached position. Thus, each cell halves receive one double strand of DNA each.
Step III : Cross-wall formation: DNA doubling is then followed by inward growth of the cell wall at mid- way between the daughter genomes. For this cell wall materials deposited between the membranes and cell is divided into two daughters.
Advantages:
(1) Only one parent is needed in binary fission .
(2) It is a rapid process.e.g. one E.coli divides in each 20 minutes .
(3) Daughter cells are identical to the parents, i.e. clones .
(4) Many daughter cells are produced in short time.e.g. one E.coli produces 4096 daughter cells in 4 hour only.
Disadvantage:
Cells produced by binary fission can not survive in changed environment due to the lack of genetic recombination.
4.2 : Budding in BacteriaBudding is a common asexual mode of reproduction in bacteria.It is explained with the following example:
Step I : In Hyphomonas polymorpha a small outgrowth forms at one end of the mother cell or on the filament. It is called the bud or the prostheca .
Step II : Simultaneously mother cell DNA replicates and segregates.
Step III: The daughter DNA and cytoplasm migrate into the nascent bud ( Jung et. al. 2019).
Step IV: The bud grows in size alongwith the emanating stalk from the mother cell, but the size of mother cell remains same.
Step V: When the bud attains about the same size of the mother cell, it separates from the mother cell [27,28] .
H.neptunium , Hypobacterium vulgare, Rhodomicrobium vannielia , etc. also perform budding.
4.3: Conidia formation in Bacteria
Many Actinomycetes, Streptomyces , etc., form chains of small , spherical spore-like conidia at the tip of aerial hyphaes. During conidia formation the aerial hyphae become coiled and septa developed at an interval of 1-2 micrometre. Simultaneously, DNA also divides and may reaches each codia before the completion of septation.
4.4: Cyst formation in Bacteria
According to Sadoff et.al., 1975 a cyst is a modified vegetative cell in the members of Azotobacter . It is resistant to desiccation but no to heat. During cyst formation a vegatative cell losses it's flagella, then attains spheroid shape and gets surrounded by an intine ( made up of lipids and carbohydrates) and a multi-layered exine( made up of lipopolysaccharide and lipoproteins) , Lin (1978).
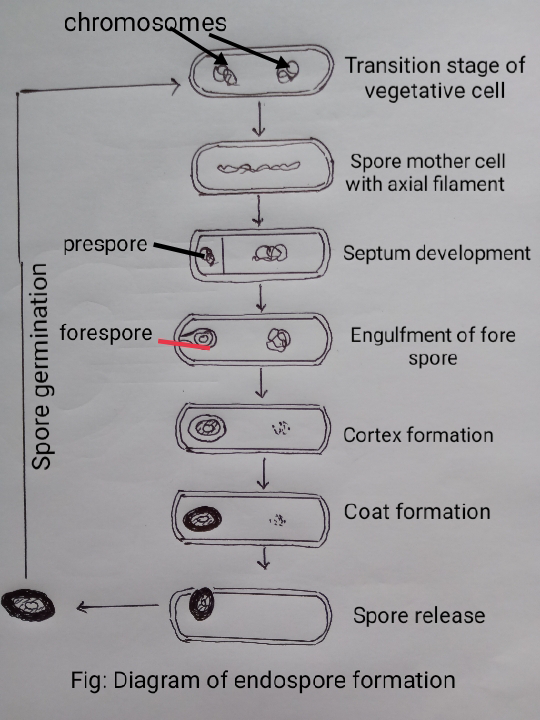
4.5: Endospore formation in Bacteria
A number of bacteria like, Bacillus, Clostridium and Thermoactinomyces form highly resistant spores within their cell called endospores during starvation and desiccation.
Ultrastructure :
(1) The central core of an Endospore is made up of nuclear body and spore cytoplasm including DNA, RNA , Ribosome and proteins.
(2) A delicate membrane of peptidoglycan surrounds the central core.
(3) A spore wall follows the membrane.
(4) The spore wall is followed by inner cortex and outer cortex .
(5) The cotrex is made up of dipicolinic acid ( DPA) , peptidoglycan and Ca (+2) ions .
(6) The cortex is surrounded by 1-2 spore coat(s) of lipids, proteins and glycopeptide.
(7) A lipoproteinous thick and wrinkled exosporium surrounds the whole structure.
Endospore formation : A vegetative cell performs the following seven stages to form an endospore:
Stage I: The two DNA of transition vegetative cell join to form a single axial filament and lies in the centre of the cell.
Stage II: A transverse septum is laid down near one end of the cell ,thus, a small prespore and a large portion are formed. Simultaneously, genome splits and attached to the mesosome.
Stage III : A plasma membrane encloses prespore to make it double membrane structure.
Stage IV : A new layer of peptidoglycan is formed in between the two membranes of prespore. This new layer turns into the germ cell wall.
Stage V: A cortex developed around the germ cell wall.
Stage VI : A thick spore coat surrounds the cortex.
Stage VII: Now the mature en dospore is released from the mother cell by autolysis .
5.Bacterial plasmid (types) and episome
5.1.Plasmid
Plasmid is an extrachromosomal circular hereditary determinants i.e.DNA, present independently the in bacterial cytoplasm , which can perform replication and transmission independently.The term " plasmid" was coined by Laderberg in 1952. Their size varies from 1.0 kb to 250 kb.
5.1.1. Classification of Plasmids:
[I] On the basis of conjugation
Bacterial plasmids are classified into two groups on the basis of the conjugation process.
[A] Conjugative plasmids : These plasmids have tra gene which makes the host bacteria to conjugate and transfer plasmid from donar to the recipient cell.
[B] Non-conjugative plasmids: These plasmids lack tra genes , hence cannot carry conjugation process between two bacteria. They may cotransfer them along with the Conjugative plasmids if present at the same host bacteria at the same time.
[II] On the basis of genes present in the plasmids: These are of five types of bacterial plasmids which are as follows:
[A] F- factor plasmids (Fertility plasmids) : These plasmids carry tra genes to make the host to be able to transfer plasmid to the recipient bacteria.
e.g. F- plasmid of E.coli.
[B] R- plasmids ( Resistance plasmids) : These plasmids carry certain genes which make the host bacteria to resist antibiotics and poisons ,like chloramphenicol, amplcillin and Hg .
e.g. plasmid RP4 of Pseudomonas .
[C] col-factor plasmids (Colicins): These plasmids have genes which make the host a killer bacteria who kills other bacteria.
e.g., plasmid ColE1 of E.coli.
[D] Degradative plasmids : These plasmids have genes within them which make the host bacteria to metabolize unwanted molecules like tolune and salicylic acid .
e.g., TOL plasmid of Pseudomonas putida L.
[E] Virulence plasmids: These plasmids carry genes which turn the host into a pathogen to others.
e.g. Ti- plasmid of Agrobacterium tumefaciens to cause crown gall of dicotyledons and Ti-plasmid.
6.Genetic Recombination: Transformation, Transduction and conjugation
6.3.Conjugation
Conjugation in bacteria is a mechanism of gene transfer from a donar F+ cell to a F- receptor cell through a conjugation tube or conjugation bridge.It was first discovered by Lederberg & Tatum in E.coli in 1946. Shigella, Salmonella, Pseudomonas, etc., also perform this mechanism.
Conjugation may takes place between (i) a F+ cell and a F- cell , (ii) Hfr cell and F- cell.
6.3.1 Conjugation between F+&F- cell
Step 1. A F+ bacterium establishes contact with the F- cell by it's sex pili.
Step 2. The sex pili then retracted within the F+ cell, hence F+ will pull F- cell.
Step 3. Then one strand of the double stranded F plasmid will break at ori and seperated from each other by the rolling circular mechanism .Then turn linier.
Step 4. Next, a nick is produced at Ori T of the F plasmid , thus a nicked single stranded DNA is formed.
Step 5. The nicking enzyme attached at the 5' end of the nicked ssDNA pushes one of the ssDNA to the F- cell through the conjugation bridge.
Step 6. Thus, both of the F+ and F- cells have single strand of F plasmid DNA. The lagging strand will remain in the donar cell and the leading strand will be in the recipient cell.
Step 7. At last , both of the strands in the respective cell perform DNA replication, thus turn dsDNA.
Thus, a F- cell receives F plasmid and turns into F+ bacteria.
7. Bacterial nutrition:
A bacterium requirs water, a carbon source, a nitrogen source and some inorganic salts as it's minimum nutritional requirements. On the basis of mode of nutrition bacteria are classified into two groups:
7.1. Autotrophic Bacteria: The bacteria that can synthesize their required organic compounds by the use of atmospheric carbon dioxide and nitrogen ,are called Autotrophic Bacteria. These autotrophs are of two types : (a) photosynthetic and (b) Chemosynthetic.
7.1.1 Photosynthetic bacteria: These bacteria do not possess chloroplasts, instead of that they have pigment bearing chromatophores of 70-300 nm. The chromatophores of green sulphur bacteria and green non-sulphur bacteria are known as chlorosomes or chlorobium vesicles. These bacteria grow in light and sulphur springs . They receive electrons from hydrogen sulphide but not from water.Hence, water is not splitted, so oxygen is not evolved. The process is anoxygenic in which they use light energy to synthesise ATP.
(a) Green Sulphur Bacteria:
These are anaerobes . Their photosynthetic pigment is chlorobium chlorophyll .
e.g. Chlorobium, Pelodictyon, etc.,
(b) Purple Sulphur Bacteria:These are aerobes. e.g. Rhodospirillum, Thiocystis, Thiocapsa, Thiospirillum , etc. Bacteriochlorophyll a & /or b is photosynthetic pigment.
7.1.2 Chemosynthetic bacteria:
These bacteria get energy from carbon dioxide or certain inorganic substances and can live in dark.
(a) Nitrogen fixing bacteria : A large number of chemolithotrophic bacteria grow in soil and water where considerable amount of ammonia released from hydrolytic break down of proteins from plants,animals and microbes. These bacteria are grouped into two heads n the basis of their mode of biochemistry:
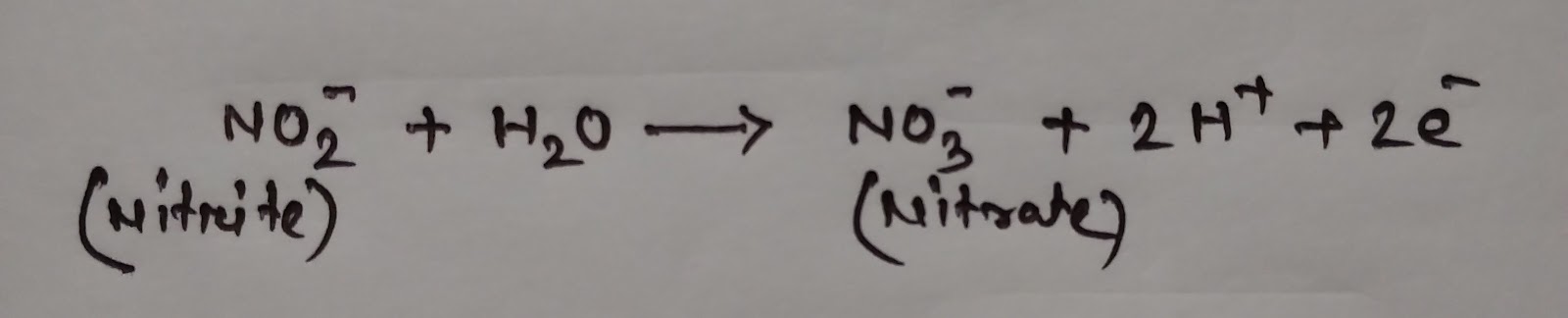
(i) Nitrosifying bacteria: Nitrosomonas, Nitrosospira and Nitrosococcus oxidise ammonia to nitrite.
These AOB (ammonia oxidizing bacteria) use 1.5 mole oxygen for the conversation of 1mole of ammonia [29] .
This process is termed as nitrosification .
(ii) Nitrosification is then followed by the process of nitrification by Nitrobacter , Nitrococcus and Nitrospira in which nitrite is converted to nitrate. These bacteria use 0.5 mole of oxygen to convert 1 mole of nitrite to nitrate [29] . These bacteria are called Nitrite- Oxidizing Bacteria (NOB).
(b) Chemosynthetic Sulphur Bacteria:
These bacteria grow on supplement of oxidisable sulphur compounds e.g. Thiobacillus neapolitanus, T. thioxidans, T.thiospora, T. denitrificans T. haplophilus and T. ferooxidans. These are gm (-) and use sulfide, elemental sulphur, thiosulphate and sulphite as the energy source e.g., Beggiatoa, oxidises hydrogen sulphide to chemical sulphur.T.thiooxidans use free sulphur to produce sulphuric acid.
(c) Iron Bacteria:
Fresh water bacteria like Sphaerotilus, Gallionella, Fertobacillus and Leptothrix oxidise ferrous ion i.e. Fe (+2) into ferric ion i.e., Fe(+3) to satisfy their energy requirement.This particular product i.e., Fe (+3) is deposited as insoluble reddish - brown ferric hydroxide, which is a gelatinous slime.They need 0.3 ppm dissolved oxygen for oxidation.
Gallionella ferruginea
(d) Hydrogen Bacteria:
A few bacteria like, Pseudomonas facilis, Nocardia opaca, Alcaligenes eutrophs ,etc., oxidise molecular hydrogen to produce water and energy.
(i) Mesophilic H bacteria : A few members of mesophilic facultative Chemolithotrophic bacteria oxidise hydrogen in presence hydrogenase enzyme to release energy. e.g. Hudrogenomonas sp.
(ii) Carboxydo H bacteria: They oxidise CO and H into carbon dioxide to release energy.
e.g., Axotobactor sp. and Hydrogenophaga sp.
(iii) Acetogenic H bacteria : Mambers of this group use H as the energy source and also utilise carbon dioxide as terminal electron acceptor to produce acetic acid.e.g., Acetobacterium woodi.
(iv) Thermophilic H bacteria: They live in hot springs and use oxygen.They assimilate carbon dioxide by TCA cycle.e.g., Hydrogenobactor sp.
7.2. Heterotrophic bacteria :
A group of parasitic, saprophytic and symbiotic bacteria obtain energy from organic sources :
(1) Parasitic bacteria: A large number of bacteria behave as causal organisms of many plant and animal disease including man also.
(2) A Large number of bacteria obtain their required energy by decomposing organic molecules and the process are termed as fermentation , putrifaction , etc. One of the familiar example is Lactobacillus.
(3) Symbiotic bacteria:
A group of bacteria grow in close association with their living host in a give and take policy.They may be ectosymbiont or endosymbiont . A common and familiar example is Rhizobium of root nodule of the leguminous plants.
8.Economic Importance of Bacteria:
(Beneficial role in Agriculture, industry, Biological control and waste management .Harmful effects: Role in food spoilage, water pollution, reduction of soil fertility, as disease causing agent).
Bacteria and Food spoilage:
(1) Bacillus cereus sometimes causes food poisoning.
(2) Milk and milk products are spoiled by a few species of Lactobacillus, Streptococcus,Proteus and Micrococcus and their exotoxins are poisonous.
(3) Clostridium botulinum produces exotoxins in food to cause "Botulism". The symptoms are swelling of tongue , double vission , respiratory paralysis, etc.
(4) Fish spoilage ": Many types of bacteria live on the surface skin, gills and alimentary canal of a living fish, but it's flesh is bacteriologically sterile . These bacteria attack the tissues of a fish after it's death. Generally, finish remain good for 14 days on ice at 0°C , then start to spoil due to microbial,chemical and physiological factors. Since these bacteria live on cold-blooded fish, they are well adapted to cold and continue to grow even in freezer [16] .
(5) Bacillus poisoning: Inadequate preservation of food may be resulted into poisoning due to the attack of Bacillus cereus which resulted in vomiting, pains and diarrhoea [17] .
(6) Inadequately cooked food, salads, drinking water,etc., are poisoned by a few spp. of Salmonella .
(7) Spoilage of Canned food:
The three most important types of canned food are (1) Flat Sour Spoilage,(2) Thermophilic Spoilage (TA) and (3) Putrefaction. The following are of some examples:
(a) Low and medium acid products , pH above 4.6, e.g. corn, peas, spinach ,asperagus............(TA) by Clostridium thermosaccharolyticim , Sulphide spoilage by C.nigrificans , Putrefactive anaerobe by C.sporogenrs and Aerobic sporeformers by Bacillus spp.
(b) Acid Products,pH below 4.6 ,e.g. Tomato juice, fruits and fruit juice........Flat sour by Bacillus thermoacidurans , Butyric anaerobes by Clostridium butyricum and Nonsporeformers by mostly lactic acid types of bacteria [19] .
Types of food spoilage (other than canned food) with some e.g. of causal bacteria [19] :
(1) Bread..... Ropy type spoilage ... Bacillus subtilis
(2) Mapple sap and syrup .....Ropy....Enterobacter aerogens.
(3) Mapple sap and syrup.....Pink.... ...Micrococcus roseus.
(4) Fresh meat..........Putrefaction........Alkaligenes, Clostridium, Proteus vulgaris, Pseudomonas flurescens
(5) Cured meat...........Souring.......... Pseudomonas, Micrococcus
(6) Cured meat.....Greening,slime.... Lactobacillus
(7) Fish..... Discoloration.... Pseudomonas
(8) Fish.... Putrefaction.... Flavobacterium
(9) Eggs ...Green rot... Pseudomonas flurescens
(10) Eggs...Colourless rots... Pseudomonas, Alkaligenes
(11) Eggs...Black rots... Proteus
(12) Concentrated orange juice..."Off " flavor..... Acetobacter
(13) Poultry..... Slime, odor.... Pseudomonas, Alkaligenes
Bacteria and water pollution:
Escherichia coli and related organisms like Streptococcus faecalis, Clostridium perfringens are termed as coliforms. Normally these bacteria are the inhabitants of the large intestine of humans and other animals,so present in feces[19] .
(1) The presence of E.coli in water indicates recent contamination from sewage or animal wastes,which may have many disease-causing organisms. E.coli 0157:H7 can infect a man from water contamination and may cause severe diarrhoea/ sometimes bloody and abdominal cramps [18] . A water is safe if there are less than four colonies of E.coli per 100 ml of water[3] .
(2) Swimming pool and surrounding areas are easily contaminated by the pathogenic microbes, specially bacteria through the discharges from eye, nose, throat, intestinal tract and also by athlet's foot, impetigo and other dermatoses.Hence, water in the swimming pool is disinfected with chlorine [19] .
Bacteria and reduction of soil fertility:
Some facultative anaerobic bacteria like Bacillus cereus , B.licheniformis, Thiobacillus denitrificans , Paracoccus denitrificans, Pseudomonas fluorescens, Ps.stutzeri,Ps.aeruginosa,etc.perform denitrification or nitrate respiration. Plants use nitrate as a source of nitrogen from the soil. During denitrification this nitrate is converted to either ammonia or nitrogen gas . Thus, soil fertility is depleted and reducing agricultural productivity .
Bacteria as disease causing agents :
(a) Bacterial diseases of Man:
(1) Bacillus anthracis causes anthrax in cattle, but can also infect man.
(2) Staphylococcus aureus causes infections in man, causing boils, abscesses, impetigo and curbuncle.This bacteria is coagulase positive , and prevents leucocytes to cause phagocytosis.
(3) Listeria monocytogenesis causes meningitis in man.
(4) Streptococcus pyogenes causes acute tonsilitis and pharyngitis and impetigo(skin infection).
(5) S. pneumoniae causes Pneumonia.
(6) Enterococcus faecalis causes UTIs and even endocarditis.
(7) Escherichia coli is normally resent in human intestine, but specific strains cause gastroenteritis and UTIs.
(8) Specific strains of Klebsiella neumoniae cause severe pneumonia, UTIs, septicaemia or diarrhoea.
(9) Salmonella typhi causes typhoid fever .
(10) S.paratyphi causes paratyphoid.
(11) S.typhimurium and S.enteridis are the cause of gastroenteritis.
(12) Shigella dysenteriae causes bacillary dysentery.
(13) Proteus mirabilis causes UTIs.
(14) Yetisiana pestis causes plague.
(15) Y. enterocolitica is the causal organism of gastroenteritis in children.
(16) Harmophilis influenzae causes meningitis.
(17) Coxiella burnettii causes Q-fever.
(18) Legionella pneumophila is the pathogen of pneumonia.[3]
(b) Bacterial disease of Plants:
Burill (1878) first reported bacterial pathogen in case of fire blight disease of pear[ 22] . Dowson (1949) classified plant diseases into four groups according to the nature of the tissues attacked and the effects produced [23,24], which are: Parenchyma diseases, Vascular diseases , Systemic diseases and Hyperplastic diseases. A few examples are as follows:
(1) Corynebacterium rependonicum is the causal organism of "Ring rot of potato".
(2) Xanthomonas citri (Hasse) Dowson causes Citrus canker.
(3) Xanthomonas oryzae is the causal organism of " Leaf blight of rice".
(4) Xanthomonas cempestris causes "Black rot of cabbage".
(5) X.campestris pv.oryzae causes "Bacterial blight of rice".
(6) X. campestris pv. oryzicola (Fang et al. 1957) causes "Bacterial Leaf Streak" in rice. It was first described in China (Fang et al,1957) [4] and in India by Srivastava et al.,1967[5] .
(6) Angular leaf spots and Black Arm of Cotton is caused by X. campestris pv. malvacerum ( F.F.Smith) Dye . It is an aerobic Gm- rod bacteria , 0.3- 0.6 micrometre x 1.3-2.7 micrometre.
(7) Wilt and Brown Rot of Potato is caused by Pseudomonas solanacearum . Brown rot of potato was the first bacterial disease recorded from India by Cappel in 1892. [6]
(8) Yellow Ear Rot or Tundu Disease of Wheat was first described by Hutchinson (1917) from Punjab in India. Clavibacter tritici is the causal organism of this disease. It is a rod-shaped baceria.
(9)Blackleg Wilt and Soft Rot of Potato is caused by Erwinia carotovora subsp. carotovora (in India) and E. carotovora subsp. atroseptica .
(10) Fire Blight of apple ( Purus malus) and pear (P. communis ) are caused by a gm- or gm variable rod bacteria , named as Erwinia amylovora.
(11) Crown Gall of Stone Fruits is caused by the bacterium Agrobacterium tumefaciens .
(12) Stalk Rot of Maize is caused by the bacterium Erwinia chrysanthemi pv. zeae .
(13) Ribbon Stunting of Sugarcane is caused by the bacterium Clavibacter xyli subsp. xyli.
(14) A mycoplasma like organism is responsible for Greening disease of Citrus ( Ghosh et al., 1971) [7] .
(15) Angular leaf spot of Tobacco is caused by Pseudomonas angulata.
(16) Wild fire of Tobacco is caused by P.syringae pv. tabaci.
9.Mycoplasma
Mycoplasma were first isolated by Nocard et al.(1898). They were first named as Pleorupneumonia - like organisms(PPLO) due to their resemblance to the pleuropneumonia agent.Because of their fungus like appearance named as " mycoplasmas".
They belong to Class Mollicutes , Edward et al. ,1967 ( translates to " soft skin"), which has one Order Mycoplasmatales having three families, each with one genus:
Mycoplasmataceae--------------- Mycoplasma
Acholeplasmataceae ------------ Acholeplasma
Spiroplasmataceae -------------- Spiroplasma
9.1.Definition:
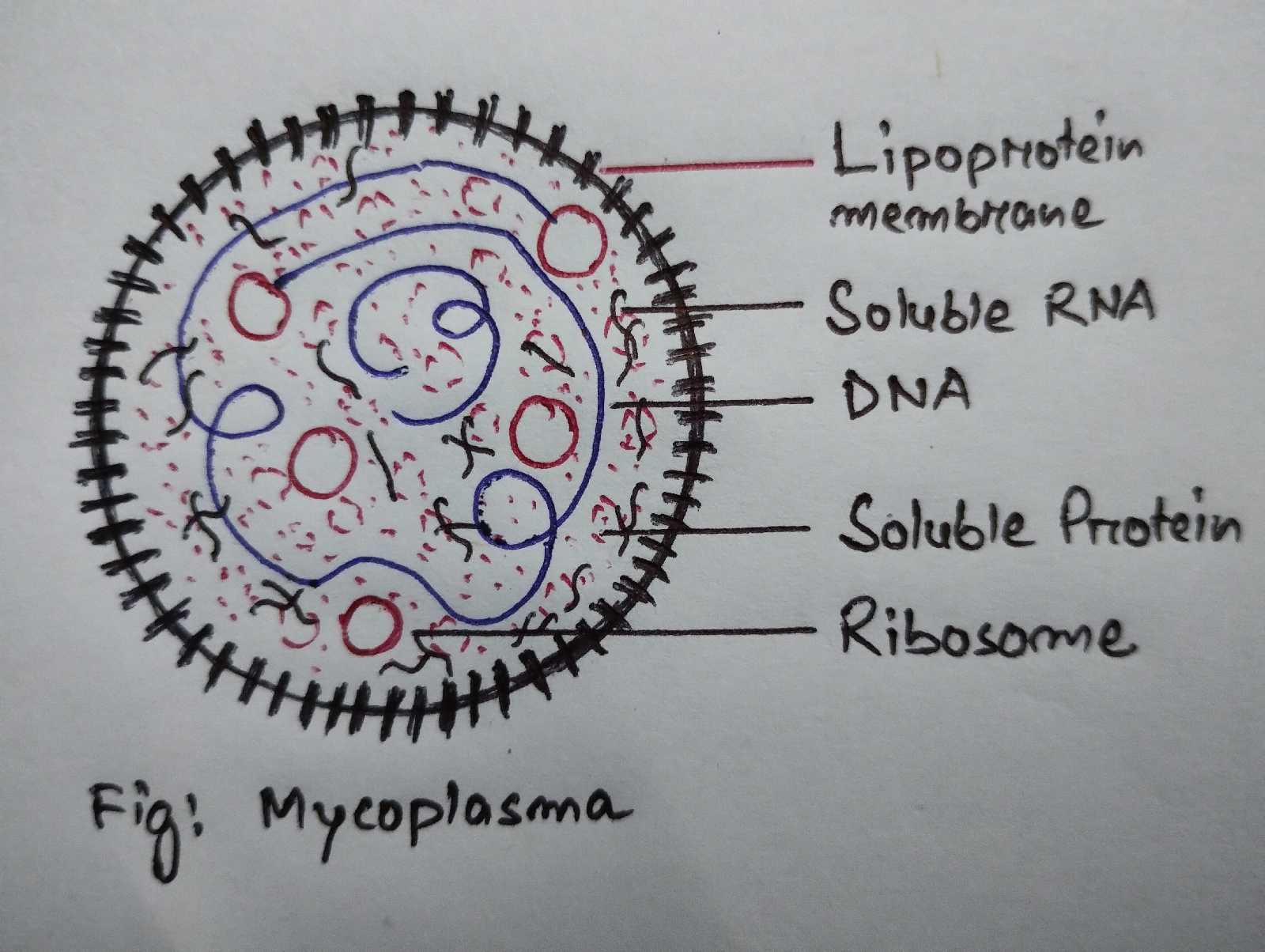
Mycoplasma are fastidious, commensal,free living or parasitic and saprophytic self replicating pleomorphic prokaryotes that lack a cellwall [6,8,9] .
9.2.Structure:
(ii) Colony diameter is about 10-15 micrometre.
(iii) The centre is round, opaque and extends into the medium.
(iv) The edge of the colony is thin and flat, forming a transparent or semi-transparent area[10].
(v) A rigid cell wall is absent in a mycoplasma . Cell wall precursors, such as diaminopimetic acid and muramic acid are also absent.
(vi) Protoplasm of a mycoplasma is bounded by a tripple layered lipoprotein "unit membrane " , which lacks mucopeptide [6,11] .
(vii) The unit membrane is about 10 nm thick .
(viii) The cell contains randomly distributed 72S ribosomes (14 nm diameter ) in the cytoplasm.
(ix) Within the cytoplasm off mycoplasma there are randomly distributed soluble RNA and a thin strand of DNA (3 nm dia.) , which has low Guanine and Cytosine as compared to bacterial DNA [6] .
(x) They are highly pleomorphic showing spherical to avoid, branched, filamentous, beaded, ringed or star shaped structure.
(xi) They measure 0.3-.08 micrometre in size.
9.3. Plant Diseases:
Mycoplasmal etiology of yellow type of disease were first suggested by Professor Asuyama et al.( Dio et al., 1967; Ishile et al.,1967) on the basis of the presence of mycoplasma like bodies (MLBs) in the phloem cells of diseased tissues [12]. Since then, more than 80 plant diseases due to MLBs have been reported from different parts of the world on the basis of the following:
(1) constant presence of MLBs in the phloem tissue of diseased plants and there complete absence in healthy plants.
(2) presence of MLBs in carrier insect vectors but absent in non-infective individuals.
(3) MLBs disappeared from the diseased tissue after the use of Tetracycline, but reappear when disease recurs.
(4) MLBs disappeared from plants and vectors when treated heat therapy [6] .
MLBs are now called as phytoplasmas and their true nature is still remain uncertain. Their genomes are only distantly related to true mycoplasma [13] ..
Suspected Mycoplasma Diseases of Plants:
Alfalfa witches' broom
Apple roliferation
Arecanut yellow leaf
Aster yellows
Bottle gourd phyllody
Blueberry stunt
Brinjal little leaf
Cassava witches' broom
Cheiranthus allioni virescence
Cherry buckskin
Citrus stubborn
Clover dwarf
Clover phyllody
Clover stolbur
Clover virescence
Corn stunt
Cotton virescence
Cowpea witches' broom
Carnberry false blossom
Crimean yellows
Cryptoyaenia witches'broom
Cucumber phyllody
Flavesvence doree (dwarf)
Giallume yellows of rice
Grassy stunt of rice
Legume little leaf
Legume witches' broom
Little cherry
Little peach
Lucrene witches'broom
Mal Azul (blue) disease of tomato
Mulberry dwarf
Papaya bunchy top
Para stolbur
Paulownia witches' broom
Pea & Green pea yellow dwarf
Peach yellows
Peanut witches' broom
Pear decline
Phloem nacrosis of elm
Phorminum yellow leaf
Pigeon pea sterility
Potato purple top
Potato stolbur
Potato witches' broom
Rice stripe
Rice yellow dwarf
Rubus stunt
Safflower phyllody
Sandal spoke
Sesame phyllody
Stolbur
Strawberry green petal
Sugarcane grassy shoot
Sugarcane white leaf
Sweet potato little leaf
Sweet potato witches' broom
Tobacco yellow dwarf
The mycoplasmal etiology of plant diseases is still not clear and Koch's Postulates have been yet to be satisfied , because plant mycoplasma culture is very difficult [14,15] .
10. Actinomycetes
10.1.Definition:
Actinomycetes are pleomorphic heterogeneous gm+ microorganisms mostly present in composts, soil, manures , water,plant remains, milk and other foods.
10.2. General characteristics :
(1) Actinomycetes are to some extent mycelial in form ,hence also termed as thread or ray bacteria.
(2) Diameter of an actinomycete cell is 1-2 micro mt.
(3) They are gm+ and non-motile but, Actinoplanes is motile [25] .
(4) Generally, they are rod shaped in a branched filament .
(5) Filaments have mumaric acid and mycolic acid is present in the call wall.
(6) They have 60-78% G+C content in DNA.
(7) They exhibit optimum growth at alkaline pH.
(8) They reproduce normally by fission, but budding, conidia and spore formation are also common .
Conidia produce singly or in straight or spirally coiled chains [25] .
10.3.Economic importance.
10.3.1.Beneficial Actinomycetes
(1) Actinomycetes degrade cellulose, hemicellulose, chitin ,etc, hence used in composting and also in bioremediation .
(2) They produce indole 3- acetic acid, siderophore and soluble phosphate which help in plant growth by protecting from phytopathogens.
(3) Actinomycetes are used to produce several antibiotics, like ,
Streptomycin from Streptomyces griseus,
Chloromyceti from S.venezualae
Aureomycin from S. aureofaciens
Rapamycin
Tetracycline
Erythromycin
Vanomycin
Rifampicin
Adriamycin
Amphotericin, etc.
(4) Actinomycetes produce several enzymes which have the following industrial uses:
Lipase : in detergents and pharmaceuticals
Cellulase : to make fodder
Catalase : in detergents
Amylase : in food, paper and textile industry
Chitinase : in Pharmaceuticals
(5) Soil fertility: Frankia , a symbiont of Alnus and Casuarina , helps to increase nitrogen content in soil 60 kg N/ha/annum to 157 kg/ ha/ annum.
(6) Secondary metabolites of Actinomycetes like AMSs prevent biocorrosion .
(7) Actinomycetes are also used as biopesticides against Musa domestica , Culex quinqefasciatus, etc.
10.3.2.Harmful Actinomycetes:
(1) Actinomyces scabies causes Common Scab of Potato.
Structure of bacteria cell
The protoplasmic content of the bacterium is confined by a 2 / 3 layered envelope: Fig: Cell wall of gm - bacteria
Fig: Cell wall of gm - bacteria
 Morphologically, a flagellum has three parts, viz.filament, hook and basal body.
Morphologically, a flagellum has three parts, viz.filament, hook and basal body.
(1)Slime layer :The outermost viscous layer of polysaccharide like dextran,dextrin and laven.When amino acids are added it turns to capsule which may be micro ( less than 0.2 micro mt in thickness)and macro (thickness is more than 0.2 micro mt ).
e.g. S strain of Diplococcus pneumoniae is capsulated while R strain is noncapsulated
(2)Cell wall: It is rigid, tough and placed just inside the slime layer/casule keeping the periplasmic space or may be the outer layer of peptidoglycan / mucopetide of (a) N-acetyl-glucosamine (NAG),
(b) N-acetyle-muramic acid(NAM) and
(c) a peptide chain of 4-5 amino acids.
Cell wall of gm- bacteria:
(1) Peptidoglycan:
(i) It is the innermost layer and constitute 5-15% of the call wall.
(ii) It is 2 nm thick and one to few layered.
(2) Periplasmic space:
(i) It is situated out side of the Peptidoglycan.
(ii) It contains proteins and oligosaccharides.
(3) Phospholipid:
(i) It is bilayered and have proteins.
(ii) A few proteins act as porins and others as target sites for antibiotics and phases.
(4) LPS:
(i) It is the principal surface antigen and the outermost layer.
(ii) It is antigen specific.
(3)Cell membrane:Semipermiable ,5-10 nm thick, elastic membrane of bilayered Phospholipid where hydrophobic are towards outside & hydrophilic are towards the inside embedding proteins here and there protecting & ensething the protoplasm of the cell is the innermost member of the envelope.
Protoplasm: It has cytoplasm with ribosome, mesosome, occasionally chromatophore , cytoplasmic inclusions ; nucleoid / genophore , plasmid .
(1) Ribosome:It is of 70S type , 10-20 nm in size (30S +50S=70S).The larger sub-unit has 1RNA molecule & 35 amino acids and the smaller one has 1 RNA & 21 aa.The ratio of RNA and protein is 2:1.Sometimes 4-6 ribosomes are placed on mRNA to make polyribosome.
(2) Mesosome: The localised infodings of cellmembrane 2 -4 per cell (more than 4 in Nitrosomonus) to participate in transverse wall formation are mesosomes (septal & lateral )
(3) Chromatophore: Photosynthetic bacteria has bacteriochlorophyll containing round (vesicle) or sac-like or tubular (lamellae) .
(4) Cytoplasmic inclusions:These are:
Lipid(poly beta-hydroxybutyrate ),
Volutin (polymetaphosphate),
Starch or glycogen(polysaccharide),and
Sulphur granules.
When volutin turns red in methyline blue it is termed Metachromatin granules .
Nucleoid/Genophore:
It may be of SS DNA or DS DNA without the nuclear membrane , nucleolus and nuclear sap .In gm+ bacteria it touches the mesosomes or in some cases plasma membrane .In E.coli genophore is super coiled . Chemically it has 80% DNA , 18-20%RNA and little proteins .
Plasmid:
It is the free extra nuclear circular DS DNA but, when integrated in genophore termed episome.It's number per bacterium is the ""copy number".e.g.
F plasmid. E.coli
R plasmid. Pseudomonas
Col plasmid. E.coli & Shigella
Virulance plasmid. E.coli
Degrataive plasmid Pseudomonas
Ti plasmid Agrobacterium tumefaciens
Flagella :
All bacteria ,usually bacills , have hair-like , whip-like locomotory organ flegella with unit protein flagellin . But all coccus and Lactobacillus,Pasteurella are Atrichous i.e. non-flagellated. Trichous types may be of polar (gm+)or non-polar:
(a) Monotrichous : single flagella at one end e.g.Vibrio cholerae
(b) Amphitrichous : flagella at the both ends .e.g. Nitrosomonus,Spirillum volutans.
(c) Cephalotrichous : 2 or more flagella at one end .e.g. Pseudomonas fluorescence.
(d) Lophotrichous: A group of flagella at one end . e.g. Spirillum serpens.
(e) Peritrichous /non-polar : Even flagellation throughout the cell .e.g. Bacillus typhosum, E.coli.
(1) Filament: A filament is external to the cell and remains connected to the hook at the cell surface.Protein fibres are the components of it and it's thickness is 13.5 nm.
(2) Hook: It is 17 nm thich and penetrates the cell wall.
(3) Basal body: (i) It joins the hook to the cell.
(ii) It attaches to the hook by a short collar.
(iii) In gm- bacteria it composed of four rings: (a) The outer L and P rings anchored to the cell wall and
(b) The inner S and M rings anchored to the cell membrane.
(iv) A rod joins the two pairs of rings and it continues to the hook.
In gm + bacteria only a single ring binds to the cell membrane.
Fimbriae /Pili:
Generally in all gm(-) rod-shaped bacteria like Shigella dysenteriae and cocci like Neisseri gonorrhoeae and few gm (+) bacteria like Corynebacterium renale have thin , short,filamentous, cylindrical hollow pili (100--500/cell) throughout the surface.A pili is made up of pillin and 1--2 micro mt in length and 3 --10 nm in diameter .
References
[1] Muriel C. van Teeseling, Miguel A. de Pedro and Felipe Cava , Determinants of Bacterial Morphology : From Fundamentals to Possibilities for Antimicrobial Targeting. Review article, Front .Microbial., 10 July 2017, Sec. Microbial Physiology and Metabolism Vol 8- 2017.
[2] Morphology of Bacteria, https:// courseware .cutm.ac.in
[3] Ajit Kr. Banerjee and Nirmalya Banerjee, Fundamentals of Microbiology and Immunology, New Central Book Agency (P) Ltd,8/1 Chintamoni Das Lane , Kolkata 700009.
[4] Fang, C.T., H.C.Ren,T.Y.Chen,Y.K.Chu,H.C.Faan and S.C.Wu(1957)," A comparison of the rice bacterial leaf blight organism with the bacterial leaf streak organism of rice and Leersia hexandra Swartz".Acta Phytopath Sinica., 3:99-124.
[5] Srivastava,D.N.(1967), Epidemiology and control of bacterial blight of rice in India.,Symp. On rice diseases and their control by growing resistant varities and other measures.Ministry of Agriculture and Forestry,Japan,Sept.25-28,A-1-A-15.
[6] R.S. Mehrotra and A.Agarwal, Plant Pathology , Second Edition, Tata McGraw-Hill, ISBN-13: 978-0- 07- 047399-7 and ISBN- 10: 0-07-047399-4.
[7] Ghosh, S.K. , S.P.Raychoudhuri , A. Verma and T.K. Naraini (1971) .Isolation and culture of mycoplasma associated with Citrus greening.Curr.Sci,40: 299-300.
[8] Jane,E.Sykrs,2014. Mycoplasma infections,Canine and Feline Infectious Diseases,pp- 382-389
[9] Christiana Maglaras ,Amie Koenig. Mycoplasma , Actinomycetes and Nocordia,Small Animal Critical Care Medicine,2nd Edition (2015), pp - 481--487
[10] Atlas of Oral Microbiology, From Healthy to Microflora to Disease,2015, pp- 95-107
[11] George H. Agrios, Plant disease caused by Prokaryotes: Bacteria and Mollicutes, Plant Pathology ,5th Edition,2005, pp 615-703
[12] Roychowdhuri S.P., Varma A. and Varma J.P. , Mycoplasma Diseases of Plants in India, Symposium on Physiology of Microorganisms pp- 437-446.
[13] Agrios,G.N.(1996), Plant Pathology ,4th Edition, Academic Press,New York,pp - 635.
[14] Ghosh,S.K.,Roychowdhuri,S.P.,Sang,A. and Varma A.(1975) Sci. and Cult. 41: 334-335.
[15] Ghosh,S.K., Roychowdhuri S.P.,Varma A.and Nariani T.K.(1971) , Curr. Sci.40: 299-300.
[16] N.Shakuntala Manay and M.Shadakshraseamy. Foods :Facts and Principles,3rd Revised Edition, 2008, New Age International (P) Ltd., Publishers.
[17] Sharma, O.P., Textbook of Thallophytes, Tata McGraw-Hill Publishing Co.Ltd,New Delhi.
[18] Bacteria , Utah Water Quality-extension. Utah State University, https://extension.usu.edu
[19] Michael J. Pelczar,JR., E.C.S. Chan and Noel R. Krieg , Microbiology,5th Edition,Tata McGraw-Hill Publishing Co.Ltd.
[20] https://www.biologyonline.com
[21] Joseph,A.,The role of Oceans in the Origin of life and in Biological Evolution. Investigating Seefloors and Oceans,2017 [ScienceDirect] .
[22] Burill,T.J.(1878).Pear blight Trans. Illinois State Hort.Soc .II; pp- 114-116.
[23] Dowson ,W.J.(1949).Manual of Bacterial Plant Diseases.Adman and Charles Black,London.
[24] Barua, H.K., Barua,P. and Barua, A. Textbook of Plant Pathology. Oxford & IBH Publishing Co.Pvt.Ltd
[25] Hawker,L.E.,Linton, A.H.,Folkers, B.F.and Carlite, M.J.(1960).An Introduction to the Biology of Micro - organisms.Efeard Arnold (Pub),London.
[26] Kleppe,R.S.OVREBO and I. LOSSIUS.1979. J. Gen. Microbiol.112: 1-13.
[27] Budding in Hyphomonas polymorpha. (Britannica ). https://www.britannica.com
[28] Hyphomonas neptunium .Max Plank Institute of Microbiology.
[29] M.Queiroz, M.V. Aun, D.M.Mortia and Alem Sobrinho, " Biological Nitrogen removal over Nitration/ Denitrition using Phenol as carbon source", Brazilian J. of Chemical Engineering, Vol 28 No 02,pp - 197-207,April- June,2011.
Unit III. Phycology I
1 History and significant contribution of Indian Phycologists:
Prof. M.O.P.Iyengar and
Prof. T.V.Desikacharya
2. General characteristics of algae- Occurence, Range of thallus organisation, Pigment types, Reserve food materials in different groups, Algal reproduction : methods of vegetative, asexual and sexual reproduction, lifecycle types
3 Outline classification of algae (Lee,1999) upto Class.
4. General characters of following algal class: Cyanophyceae, Chlorophyceae, Xanthophycese, Bacillariophyceae, Pharophyceae, Rhodophyceae
5. Economic importance of Algae
Unit IV Phycology II
1. Structure and lifecycle of the following algal genera:
Nostoc
Oedogonium
Chara
Vaucheria
Ectocarpous
Polysiphonia
2. Diatoms: Cell structure, reproduction and economic importance .
2.2.Range of thallus structure in Algae
Algae show unicellular to multicellular,simple to complex and few micron (1x1.5) very large sized thallus organisation(60-80 mt) .There are six types of algal thallus:
(1) Unicellular Thallus: The thallus is made up of only one cell and may or may not be motile.
(i)Motile form: They possess either rhizopod or flagella. In rhizopodal one the rigid cell wall is absent , so many amoeboid projections help in motility ,e.g., Chrysamoeba of Chrysophyceae. In the members of Chlorophyceae, the flagella length is equal,e.g., Chlamydomonas, but in case of Xanthophyceae or in Dinophyceae flagella length are unequal, e.g., Peridium.
Fig: Chrysamoeba A. Flagellated
B. Amoeboid
(ii) Non motile form: In case of Spirulina of Cyanophyceae the only cell of the thallus is spiral ,but in case of Chlorella , Gloeocapsa, etc. of the family Chlorophyceae the cell is spherical.
Fig: Unicellular non-motile thallus
(2) Multicellular colonial coenobium like form: A definite numbers of unicellular units connected to each other to form a colonial thallus having definite shape called coenobium which may be motile. e.g. Volvox of Chlorophyceae,Gonyaulax of Dinophyceae, etc.Fig: Gonyaulax pacifica
(3) Multicellular colonial aggregated form : The number of the cells in the colony is indefinite but the aggregation is irregular.These are of three types:
(i) In Palmelloid type a group of few non motile cells remain embedded in amorphous gelatinous or in Mucilage matrix.e.g., Chlamydomonas.
(ii) In Dendroid form microscopic plant body looks trees ,e.g.Prasinocladus, Ecballocystopis,etc.
(iii) In Rhizopodal type cells are attached to each other by rhizopoda.e.g., Chrysidiastrum.
(4) Filamentous thallus : The constituting cells of the thallus attached end to end, thus uniseriate and branched or unbranched structure is formed.
(a) A large number of Chlorophyceae like Spirogyra , Ulothrix, Oedogonium,etc and Nostoc, Anabaena,etc of the members of Cyanophyceae exhibit unbranched -uniseriate filament.
(b)The uniseriate branched filamentous thallus are of three types:
(i) In the simple form branches may arise from any cell except the basal cell.e.g., Cladophora.
(ii) In the Heterotrochous type many erect uniseriate filaments arise from the prostate filaments .e.g., Ectocarpous.
(iii) In case of Siphonous form the filament is non septate and coenocytic having branches. e.g., Vaucheria.
(5) Pseudoparenchymatous type: One or more axial filaments in close juxtaposition gives an uniaxial form ( e.g. Batrachospermum) or a multiaxial thallus e.g., Chodium, Chondrous , etc.
(6) In case of Perenchymatous thallus numerous septation make it large sized.
2.3. Pigment System in algae
Pigments play vital role in algal taxonomy. There are four different kinds of pigments in algae:- Chlorophylls, carotenes, xanthophylls & phycobilins. Round (1973) recognised :-
5 chlorophylls, 5 carotenes, 20 xanthophylls and 6 phycobilins in algae.
Cyanophyceae
Pigments include chl a (green), beta-carotene, xanthophylls, c-phycoerythrin (red), c-phycocyanin and allophycocyanin.
Chlorophyceae
Dominant pigments are chl a, chl b, alpha carotene, beta carotene, xanthophylls.In Siphonales siphonein and siphonoxanthin present.
Xanthophyceae
Main pigments are chl a, chl c, chl e, xanthophyll, beta carotene.
Bacillariophyceae
Chl a, chl c, beta-carotene and xanthophylls like fucoxanthin, diatoxanthin and diadinoxanthin are the main photosynthetic pigments.
Phaeophyceae
Chl a, chl c, beta-carotene, fucoxanthin & vioxanthin are present in the chromatophores.
Rhodophyceae
Red pigments r-phycoerythrin & r-phycocyanin,chl a, chl d and xanthophyll ( taraxanthin) are the chief pigments.
2.4.Reserve food materials in algae:
Cyanophyceae
Reserve food materials are cyanophycean starch and myxophycean starch, glycogen, metachromatin and fats.
Chlorophyceae
Reserve food is starch ( amylose and amylopectin) , but some have oil.
Xanthophyceae
Reserve food materials are oils and leucosin.
Bacillariophyceae
Oil, volutin and chrysolaminarin or chrysose are the reserve food materials.
Phaeophyceae
Laminarin and mannitol are the reserve food materials.
Rhodophyceae
Floridean starch, floridoside and mannoglycerate are the reserve food materials.
3.Classification of Algae(Lee,1999)
The standard botanical classification system is used in the systematics of the algae:
Phylum- phyta
Class- phyceae
Order- ales
Family- aceae
Genus
Species
Lee divided algae into 4 groups
Group 1: Prokaryotic algae
Phylum: Cyanophyta:
Prokaryotic algae
Pigments chl a; phycoboliproteins.
Class: Cyanophyceae (blue green algae)
Group 2: Eukaryotic algae with chloroplasts surrounded by only two membranes of the chloroplast envelope.
Phylum: Glaucophyta:
Algae that represent an intermediate position in the evolution of chloroplasts; photosynthesis is carried out by modified endosymbiotic cyanobacteria.
Phylum: Rhodophyta:
chl a, phycoboliproteins, no flagellated cells, storage product is Floridean starch.
Class: Rhodophyceae (red algae)
Phylum: Chlorophyta:
Chl a and chl b, storage product starch is found inside the chloroplast.
Class: Chlorophyceae (green algae)
Group 3: Eukaryotic algae with chloroplasts surrounded by one membrane of chloroplast endoplasmic reticulum.
Phylum: Euglenophyta:
Chl a and chl b, one flagellum with a spiraled row of fibrillat hairs, proteinaceous pellicle in strips under the plasma membrane, storage product is paramylon, characteristic type of cell division.
Class: Euglenophyceae (euglenoids)
Phylum : Dinophyta:
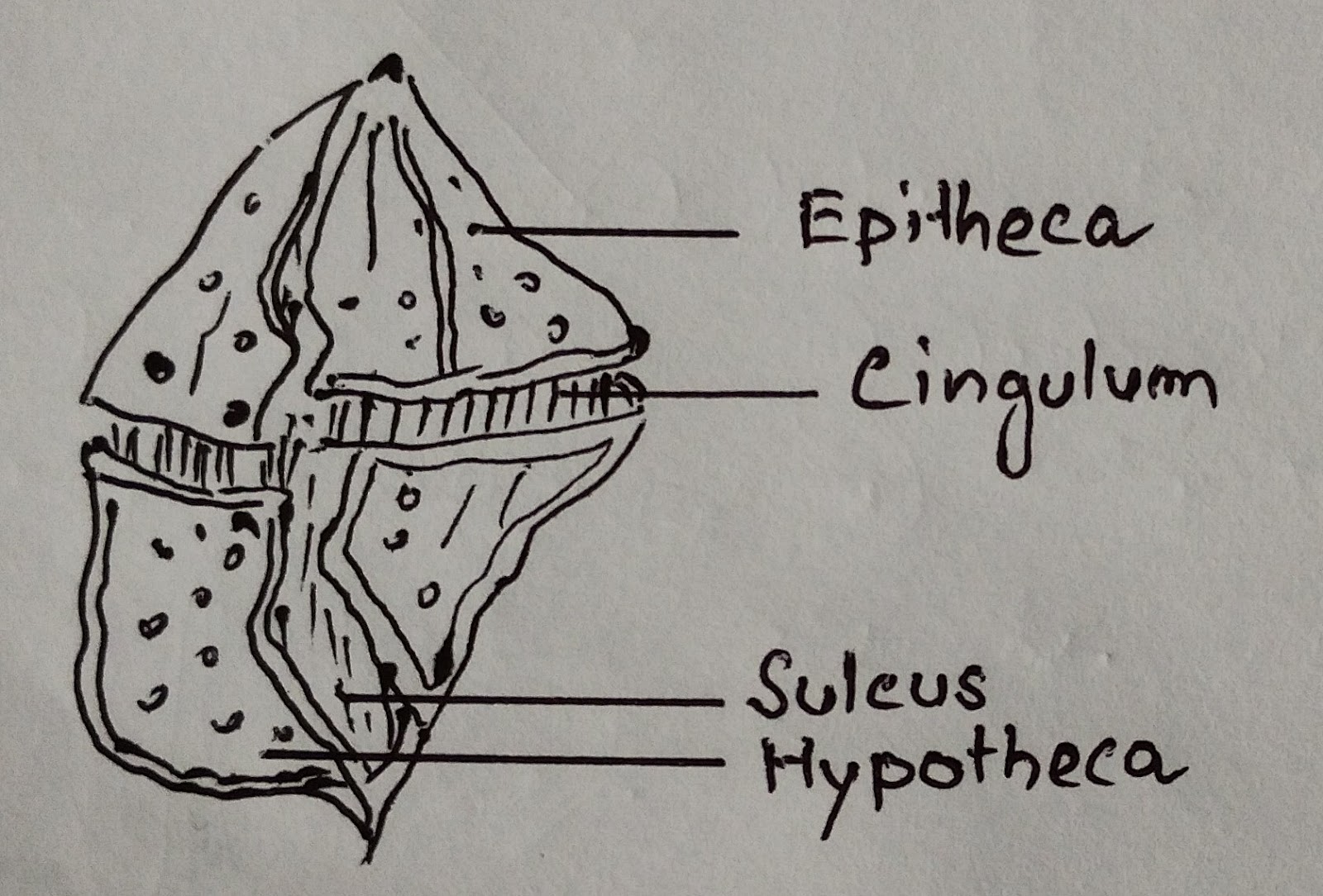
Mesokaryotic nucleus; chl a and chl c¹; cell commonly divided into an epicone and a hypocone by girdle; helical transverse flagellum; thecal plates in vesicles under the plasma membrane.
Class : Dinophyceae ( dinoflagellates)
Phylum: Apicompexa:
Heterotrophic flagellates with colourless plastids.
Group 4: Eukaryotic algae with chloroplasts surrounded by two membranes of chloroplast endoplasmic reticulum.
Phylum: Cryptophyta:
Nucleomorph present between inner and outer membrane of chloroplast endoplasmic reticulum; starch formed as grains between inner membrane of chloroplast endoplasmic reticulum and chloroplast envelope; chl a and chl a, phycoboliproteins; periplast inside plasma membrane.
Class: Cryptophyceae (cryptophytes)
Phylum: Heterokontophyta:
Anterior tinsel and posterior whiplash flagellum; chl a and chl c ; fucoxanthin; storage product usually chrysolaminarin in vesicles.
Chrysophyceae
Synutophyceae
Eustigmatophyceae
Pinguiophyceae
Dictyochophyceae
Pelagophyceae
Bolidophyceae
Bacillariophyceae
Raphidophyceae
Xanthophyceae
Phaeothamniophyceae
Phaeophyceae
Phylum : Prymnesiophyta
Two whiplash flagella; haptonema present; chl a and c ; fucoxanthin; scales common outside cell; storage product chrysolaminarin occuring in vesicles.
4. General characteristics / salient features of different classes of algae
4.1. Cyanophyceae
(1) Freshwater, terrestrial, epiphytic, endophytic and symbiotic.
(2) Thallus ranges from unicellular to filamentous.
(3) Cell structure prokaryotic, presence of primitive type of nucleus, called central body , which lacks a nucleolus and a nuclear membrane.
(4) Mucopeptide and muramic acid are present in cell wall.
(5) Flagella completely absent .
(6) Pigments include chl a (green), beta- carotene, xanthophylls, c-phycoerythrin (red), c-phycocyanin (blue) , and allophycocyanin.
(7) Reserve food materials are cyanophycin and myxophycean starch , glycogen, metachromatin and fats.
(8) Mucilage is secreted by all .
(9) Heterocyst present.
(10) Reproduction is asexual .Sexual methods are completely absent (Fritsch, 1945; Smith, 1955).
e.g., Nostoc, Anabaena, Spirulina, Oscilatoria,etc.
4.2. Chlorophyceae
(1) Freshwater,marine and terrestrial.
(2) Thallus unicellular motile, coenobial, colonial, non motile thallus, filamentous or siphonaceous.
(3) Cell eukaryotic.
(4) Cell wall have cellulose and pectin.
(5) Number of flagella 1,2-8 or many and apical or subapical and equal in length.
(6) Dominat pigments are chl a , chl b, alpha & beta carotene and xanthophylls. In Siphonales siphonein and siphonoxanthin present.
(7) Reserve food is starch ( amylose & amylopectin), but some have oil.
(8) Pyrenoids present in chloroplast.
(9) Sexual reproduction isogamy to oogamy.
(10) Meiosis zygotic and life cycle haplontic.
e.g. Chlamydomonas, Volvox, Chlorella, Oedogonium, etc.
4.3. Xanthophyceae
(1) Mostly freshwater, a few marine.
(2) Thallus motile, palmelloid, filamentous and even siphonous.
(3) Cell eukaryotic.
(4) Cell wall is made by cellulose.
(5) Motile cells with two enequal anterior flagella , one pleuronematic and other acronematic.
(6) Main pigments are chl a, chl c, chl e, xanthophyll, beta carotene .( Due to the presence of excess of yellow xanthophylls they are called yellow -green algae.
(7) Reserve food is oil and leucosin .
(8) Pyrenoids are either rare or totally absent.
(9) Reproduction is asexual, rarely sexual ( isogamy to oogamy) .
(10) All members are haploid.
e.g. Heterochloris, Botrydium, etc.
4.4. Bacillariophyceae
(1) Freshwater as well as marine.
(2) Thallus unicellular,free or colonial with radial or bilateral symmetry.
(3) Cell eukaryotic and diploid.
(4) Silica and pectin present in the two halves of cell wall.
(5) 1-2 anterior pentonematic flagella present on the motile cells.
(6) Chl a, chl c, beta - carotene and xanthophylls like fucoxanthin, diatoxanthin and diadinoxanthin are the main photosynthetic pigments.
(7) Oil, volutin and chrysolaminarin or chrysose are the reserve food materials.
(8) Vegetative reproduction by cell division and sexual reproduction is isogamous with the formation of auxospores .
(9) Meiosis gametogenic.
(10) Lifecycle monogenic and diplontic.
e.g., Cyclotella, Synedra, etc.
4.5. Phaeophyceae
(1) 99.7% are are marine (Chapman and Chapman, 1973).
(2) Thallus multicellular, filamentous and branched. Unicellular, colonial and unbranched structure are completely absent.
(3) Cells eukaryotic.
(4) Cell wall contains cellulose, fucinic acid and alginic acid.
(5) Motile cells have two enequal lateral flagella, one tinsel type and other whiplash type.
(6) Chl a, chl c, beta-carotene, fucoxanthin and vioxanthin are present in chromatophores. Thallus appears brown due to fucoxanthin .
(7) Laminarin and mannitol are the reserve food materials.
(8) Sexual reproduction in isogamy to oogamy.
(9) Motile reproductive bodies formed either in the unilocular or plurilocular sporangia.
(10) Life cycle monogenetic (diplontic) or digenetic (isomorphic or heteromorphic ) (Fritsch, 1945).
e.g., Ectocarpous,Laminaria, etc.
4.6. Rhodophyceae
(1) 98% are marine (Chapman and Chapman, 1973).
(2) A few are unicellular, but mostly filamentous, pseudoparenchymatous or parenchymatous.
(3) Cells eukaryotic.
(4) Cellulose, pectin and polysulphate esters are present in cell wall.Pit connections present.
(5) Flagella completely absent.
(6) Red pigments r-phycoerythrin & r-phycocyanin , chl a , chl d and xanthophyll ( taraxanthin) are the chief pigments.
(7) Floridean starch, floridoside & mannoglycerate are the reserve food materials.
(8) Sexual reproduction is oogamous. Male sex organ is spermatangium and female sex organ is carpogonium. Zygote never released from the carpogonium ( Fritsch, 1945).
(9) The fruit body, carposporophyte formed due to the post fertilization events.
(10) Most members show biphasic or triphasic life cycle ,e.g.Porphyridium (unicellular), Polysiphonia, etc (Dixon,1973).
Unit 4 Phycology II
4.1. Nostoc
4.1.1. Systematic Position
According to Lee(1999)
Phylum Cyanophyta
Class Cyanophyceae
Order Nostocales
Family Nostocaceae
Genus Nostoc
4.1.2. Nomenclature
The term " Nostoc" was first used by Paracelsus ( Bold and Wynne,1978). It is also called " Star Jelly", "Witches'butter", " Dragon Don't" , " Ground boogers", "Mere's egg".
4.1.3. Occurrence
The range of distribution of Nostoc is polar to tropical. Nostoc is an fresh water , subaerophytic , endo-symbiotic or terrestrial alga. N. punctiforme is an endo- symbiont within the corralloid roots of Cycas, Anthoceros thallus, roots of Zamia , within the fungus Geosiphon pyriforme and underground stem of Gunnera manicata. A few species like N.sphaericum, N.collema are common phycobiont .
4.1.4.Indian Species
N. punctiforme, N. endophytum, N rivulare, N.calcicola, N. ellipsoporum, N.muscorum, N.sphericum , N. hatei , etc.
4.1.5. Types
Different spp. of Nostoc are classified as:
(1) the commune type, where the colonies have a firm pellicle ,
(2) the piscinale type,where the firm pellicle absent, and
(3) the punctiforme type,where filamentous organisation is lost and aseriate form results.
Colony: (i) A Nostoc colony is ball like and enveloped by a gelatinous sheath.
(ii) The balls are bluish green in colour.
(iii) Thousands of twisted or contorted trichomes aggregate to form a colony.
(iv) There is a constriction between two adjacent cells of a Nostoc filament, hence, appeared beaded or moniliform.
Trichome: (i) An unbranched, uniseriate, contorted or twisted beaded structure is a trichome.
(ii)A mucilagenous hyaline or coloured sheath is present around each trichome.
Heterocyst : (i) A Nostoc filament has some large, oval or spherical colourless empty cells called as Heterocyst.
(ii) Generally Heterocyst are intercalary in position, but in N.lincia position is terminal.
(iii) The inner layer of cellulose thickened at the two poles of a Heterocyst to make polar nodules.
(iv) A narrow pore is present within a polar nodule.
Cell structure: (i) A Nostoc cell is prokaryotic, oval or spherical having a plasma membrane and cellolose cell wall.
(ii) Centroplasm and chromoplasm combine to form the Protoplasm.
(iii) Chromoplasm contains pigments and reserve food.
(iv) Genetic materials are confined in the incipient nucleus (without nucleolous & nuclear membrane ).
4.1.7 Reproduction
Nostoc reproduces only by vegetative methods. No sexual reproduction is observed at all.
Vegetative reproduction:
Nostoc reproduces vegetatively by the following methods:
(i) By fragmentation: A Nostoc colony may breaks into two to many fragments due to mechanical or physiological factors.Latter each fragment turns to a new Nostoc colony.
(ii) By Hormogonia :
(a)When an intercalary cells of a Nostoc filament degraded , the filament results into two to many filaments.
(b) Each filament then termed as Hormogonia .
(c) Now, each Hormogonia comes out of the sheath of the colony and grow rapidly into new colonies.
(d) If hormogonias fail to come out, then resulted to many trichomes within the old colony.
(iii) By akinites :
(a) These are the resting spores of Nostoc during unfavourable conditions.
(b) An akinite has an additional three layered coat around the cell wall.
(c) Protoplasm accumulates reserve food.
(d) Generally a vegetative cell of adjacent to the heterocyst first metamorphoses to an akinite, then the rest cells present in between two heterocysts.
(e) During favourable conditions Protoplasm of an akinite becomes active and breaks outer wall and forms a new trichome.
(iv) By Heterocyst: In N. commune heterocysts divide into 4- celled germling, then each germling ruptures the outer wall and develops into a new filament.
(v) By Endospore: The endospores of N.commune and N.microscopicum germinate into new filaments in favourable conditions.
Exception: Different filaments of N. muscorum fuse or anastomose to each other and this method may be compared with somatogamy.
4.1.8. Economic importance of Nostoc
1. Chryptophycin 1, derived from Nostoc ATCC53789 is a strong fungicide.
2. Cryptophysin 1 derived from Nostoc sp. GVS 224 is cytotoxic against human tumour cell lines.
3. Trypsin inhibitor Nostosin A and B are trypsin inhibitor and obtained from Nostoc sp. strain FSN (B.Nowruzi,2018).
4.2.Oedogonium
4.2.1. Systematic Position
Division --- Chlorophyta
Class----- Chlorophyceae
Order---- Oedogoniales
Family ----- Oedogoniaceae
Family---- Oedogonium
4.2.2. Occurrence
Oedogonium is exclusively freshwater alga . It's filament attaches to the underwater wood,stem, leaves and stone with the help of basal holdfast. Oedogonium terrestrial, Oedogonium randhawe etc. are terrestrial and grow on moist soil.
4.2.3. Indian Species
More than 200 species of Oedogonium are found in India e.g., O.areolatum, O.armigerum, O.cardiacum , O. aster and O.elegans.
4.2.4. Thallus structure
(i) Oedogonium is a green , multicellular, unbranched filament with many cylindrical uniceriate cells with different size and shape.
(ii) According to position cells are of three types:-
(a) Basal cell
(b) Apical cell, and
( c) Intercalary cells
(iii) Basal cell or holdfast: It is colourless.It's lower part has multilobed or disc like projections to hold the substratum.The upper part of the cell is broad and rounded.
(iv) Apical cell: It's apex is rounded or acuminate .It is green in colour.
(v) Intercalary cells: These cells lie between basal cell and apical cell.All cells are green , most of them are cylindrical, but a few have ring like caps at their upper end ( the number of caps of a cell indicate the number of times the cell has divided).A few such cells converted to reproductive cells like antheridia, oogonia and androsporangia.
4.2.5. Cell Structure
A typical cell consists of:
(1) Cell wall: Cell wall is rigid and differentiated into three layers: (a) outer most layer of chitin, (b) middle layer of pectin, and (c) inner most layer of cellulose .
(2) Plasma membrane: It is present just inner to the cell wall and encloses cytolasm and nucleus.
(3) Nucleus: The nucleus lies centrally , sometimes eccentric, and embedded in cytoplasm just within the chloroplast.
(4) Chloroplast: It is large , reticulate and parietal in position extended from one end to the other. There are many pyrinoides in it. According to Hoffman's a chloroplast contains many microtubules.
(5) Central vacuole: It remains filled with cell sap.
(6) Others: Other cell organelles ,like, mitochondria, Golgi bodies, endoplasmic reticulum,etc., are also present in each cell.
4.2.6. Cell Division
Oedogonium grows due to the division of intercalary cells.These cells divide by the following ways:
(1) Peripheral nucleus moves towards the centre and slightly towards the upper side of the cell.
(2) Then a ring-like thickening forms in the upper part of the cell, and gradually increase in thickness.
(3) The ring turns to a groove and simultaneously nucleus starts mitosis.
(4) After the complete nuclear division a row of microtubules formed in between two nuclei to form the septum ,which at first floats in the cell . Later this septum migrates to the base of daughter,i.e. to the ring and gradually attaches to the lateral wall .
(5) The gradual increase of the ring causes the split of mother cell wall and a part of ruptured parent wall remains attached to the anterior end of the newly formed daughter cell as a cap,while the other part attaches to the proximal end. Hence, newly formed daughter cell remains interposed between two old parts of the parent cell.
4.2.7 Reproduction
Oedogonium reproduces by vegetative, asexual and sexual methods.
Vegetative reproduction :
(1) Fragmentation: A filament of Oedogonium may divides or fragmented by:
(i) drying up or dehydration of intercalary cells,
(ii)convertion of intercalary cell into sporangia,
(iii)accidental breaking of the filament.
(2) Akinetes: According to Handa,1928 a chain of oval or rounded thick walled, reddish-brown akinetes formed during unfavorable time and on the onset of favorable conditions each one akinete germinaes into a new filament.
Asexual reproduction: Oedogonium reproduces asexually by zoospore, which is discussed as the following:
(i) Any cap cell, generally recently divided, acts as a zoosporangium.
(ii) First, the protoplast of the zoosporangium contracts from the cell wall.
(iii) Nucleus of that cell moves towards one side and whole protoplast turns round/oval.
(iv) A lens-shaped hyaline area developed on one side of the protoplast, just close to the nucleus.
(v) A ring of basal granules ,each with a single flagellum, appears around the hyaline area.
(vi) These granules are interconnected by a fibrous strand( Ringo,1967).
(vii) A mature zoospore liberates from the zoosporangium by rupturing near apical cap.
(viii) After 3-10 minutes of liberation the thin mucilaginous vesicle present around the zoospore dissolves and the zoospore swims in water.
(ix) After about one hour swimming, it's anterior end attached to the substratum and becomes deflagellated and turns to elongate.
(x) The lower part turns to holdfast and the upper part divides repeatedly to form a new filament.
Sexual Reproduction:
Oedogonium reproduces by advanced oogamy. Male and female gametes produced in antheridia and oogonia respectivelyand both the gametes differ morphologically as wall as physiologically.
Distribution of Sex organs:
Oedogonium species are divided into Macramdrous and Nannandrous type on the basis of distribution of sex organs:
(a) Macramdrous species: In these species antheridia occur on the filaments of normal size.They may be homothallic or heterothallic. O. nodulosum and O. fragile the male and female sex organs are found on the same filament, hence Homothallic Macramdrous. But in case of O.crassum and O. aquaticim antheridia and oogonia
References
Bold, H.C. and M.J.Wynne(1978) , Introduction to the Algae: Structure and Reproduction, Prentice-Hall of India ,New Delhi.
Chapman ,V.J. and D.J. Chapman (1973), The Algae, The Macmillan Press Ltd, London.
Dixon,P.S. (1973),Biology of Rhodophyceae, Hafner Press,New York.
Fritsch,F.E.(1945),The Structure and Reproduction of Algae, Vol.II, University Press , Cambridge.
Hoffman,L.R.(1967), Observations on the fine str. of Oedogonium, J.Phycol.3:212-221.
Nowruzi. B, Haghighat. B., Fahimi. H. , Mohammadi. E., Journal of Pharmaceutical Health Service Research, vol 9, Issue 9, March 2018,pp 5-12.
Round, F.E.(1973), The Biology of the Algae ,2nd edn, Edward Arnold, London.
Smith,G.M., Cryptogemic Botany,Vol.I., 2nd Edition,McGraw Hill Book Co.,Inc.New York,1945.























A healthy lifestyle is the ultimate form of self-respect
ReplyDeleteIv therapy near me in Winchester